![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


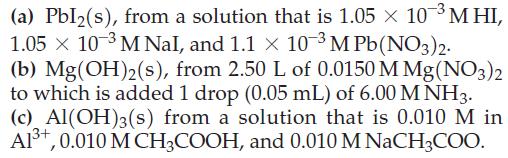
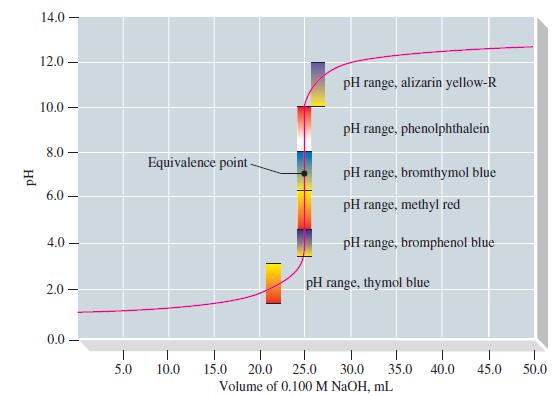
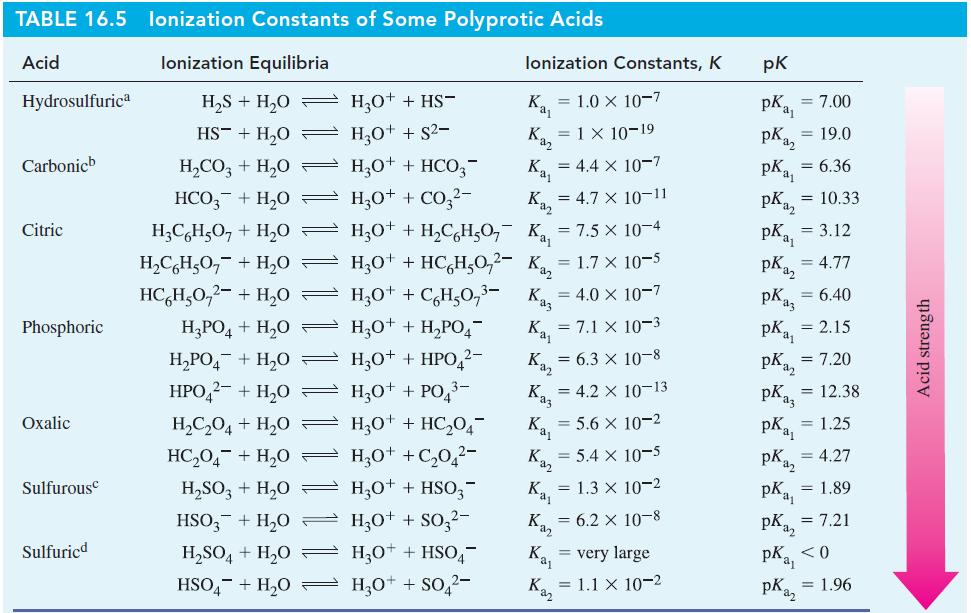
![(a) [HSO3] = 0.013 M; (b) [SO3] = 6.3 108 M; (c) [H3O+] = 0.10 M; (d) [H3O+] = 0.013 M; (e) [SO3-] = 0.036 M.](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/5/6/8/170655c9c6ae4b521.png)
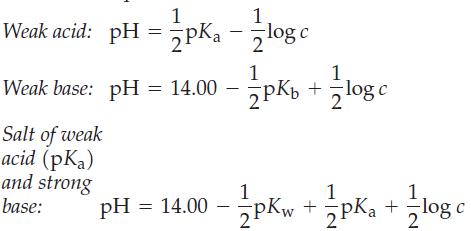
![CHCHCOH + HO H3O+ + CH5CHCO Ka 4.9 x 10-5 = (a) What is [C6H5CHCO2] in 0.186 M C6H5CHCOH? (b) What is the pH](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/5/5/4/845655c685dba76b1700554844425.jpg)
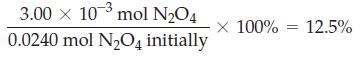
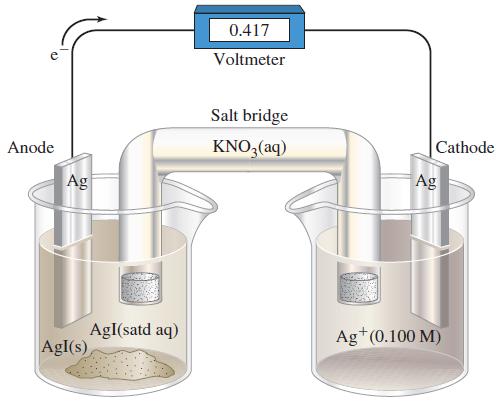
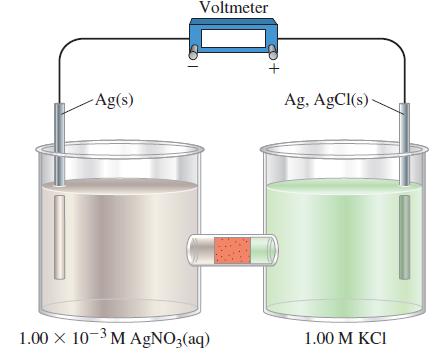
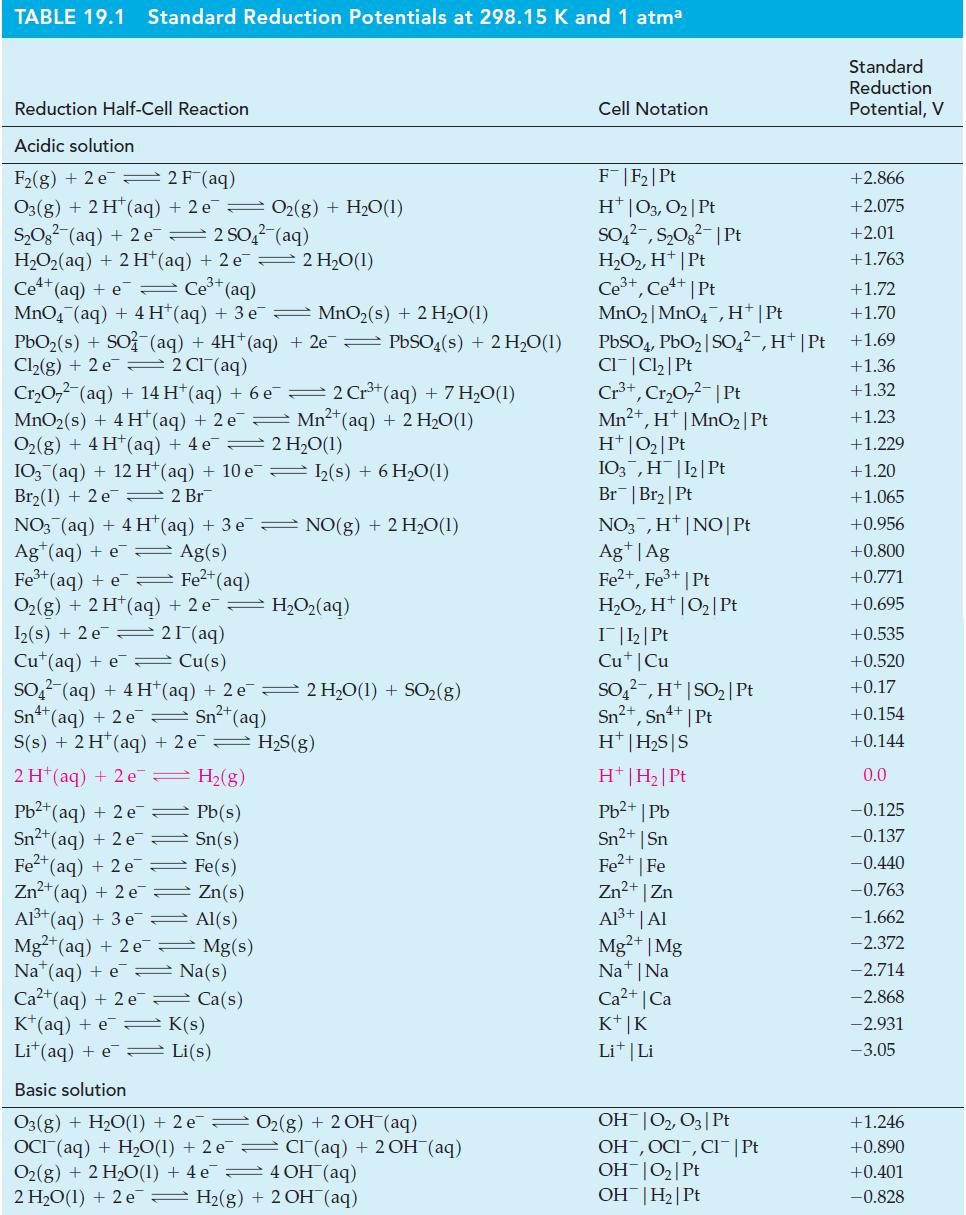
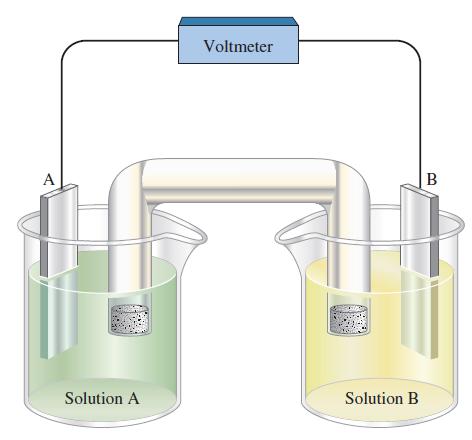

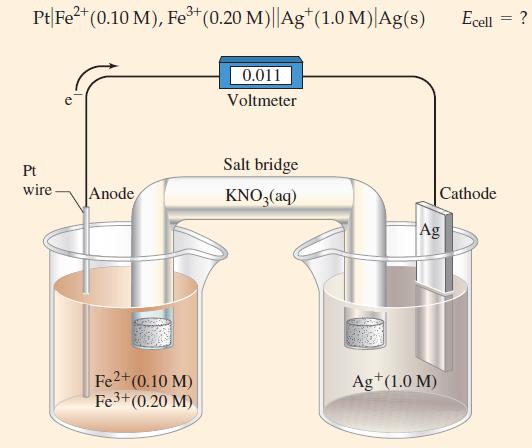
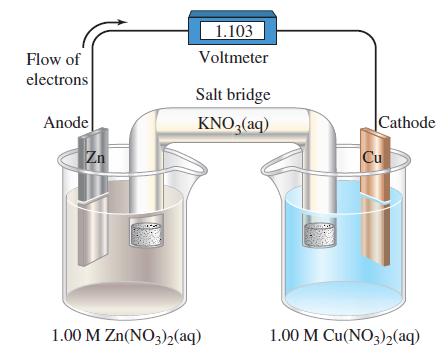
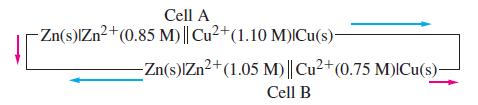
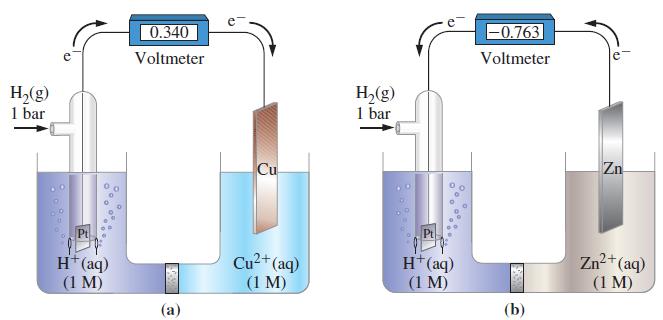
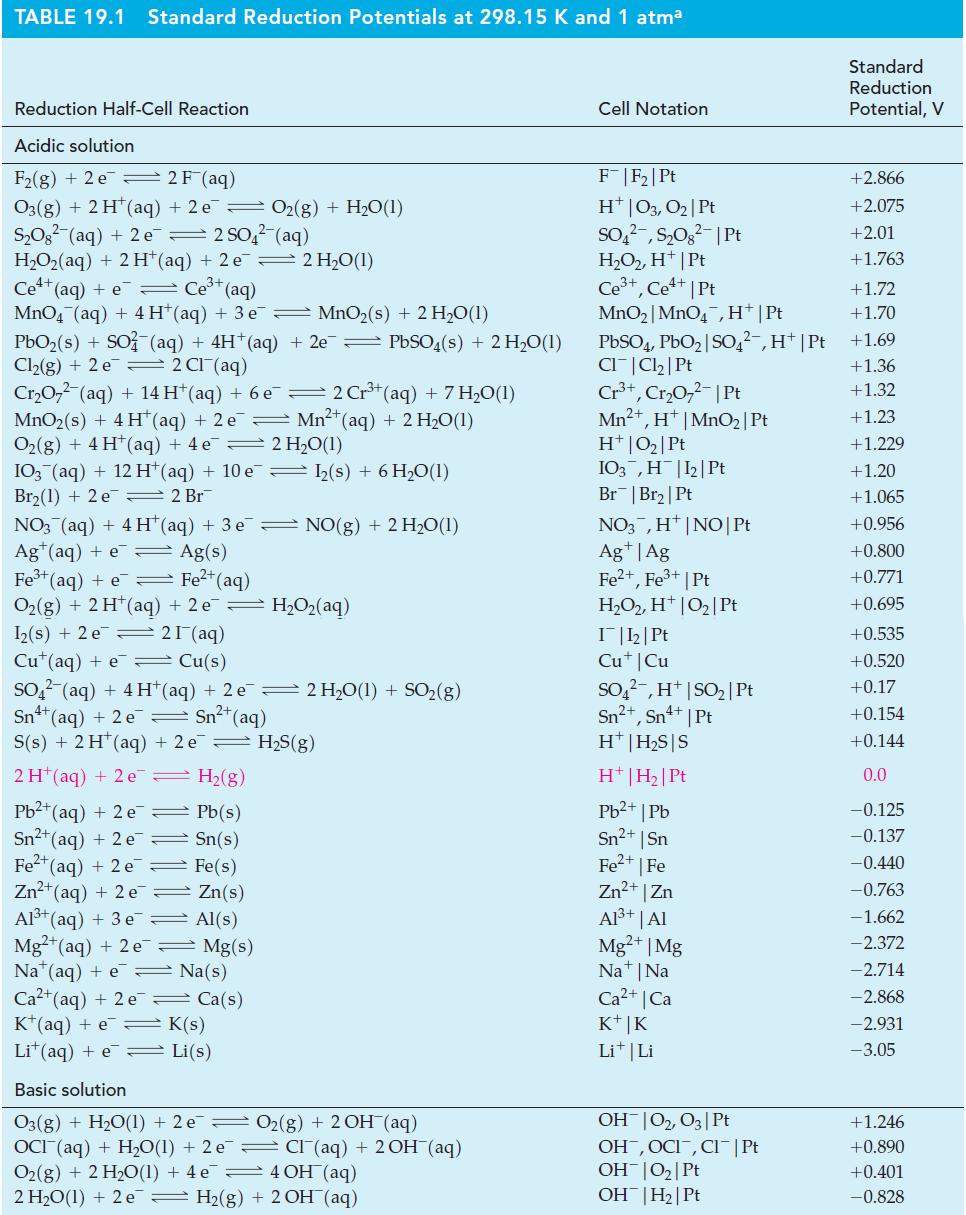
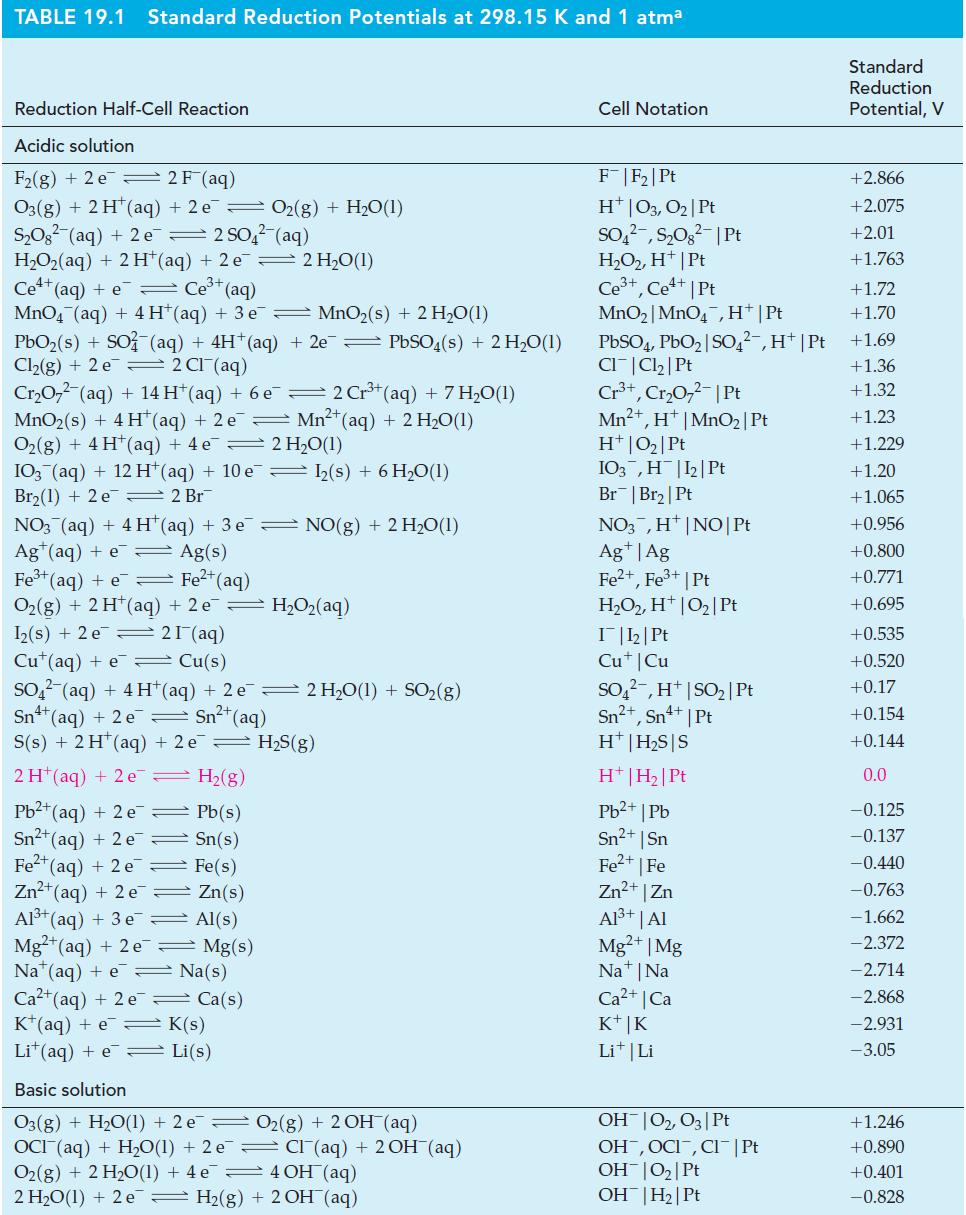
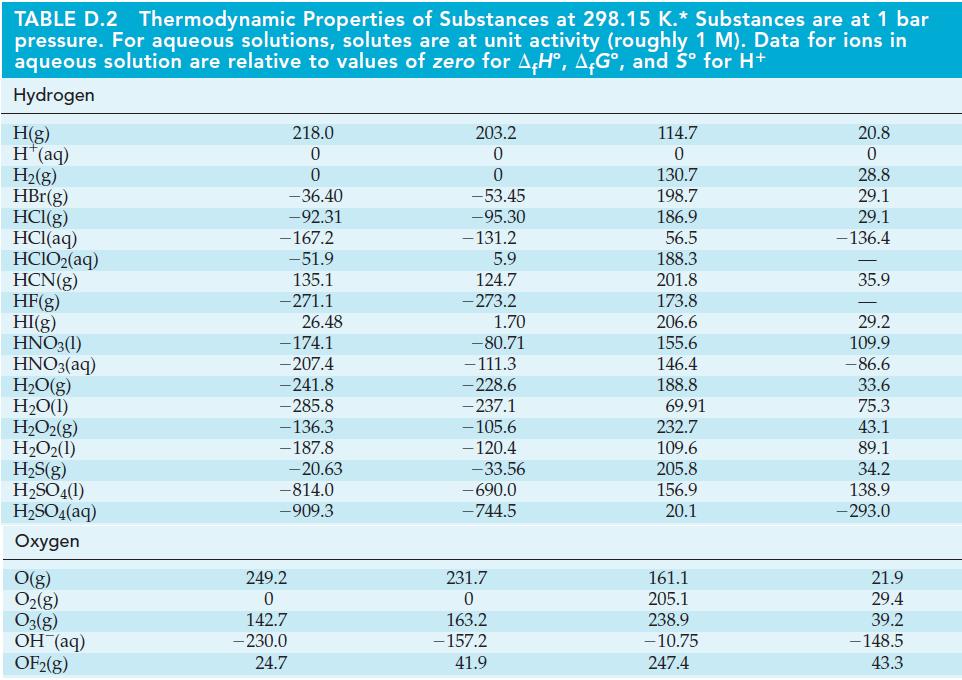
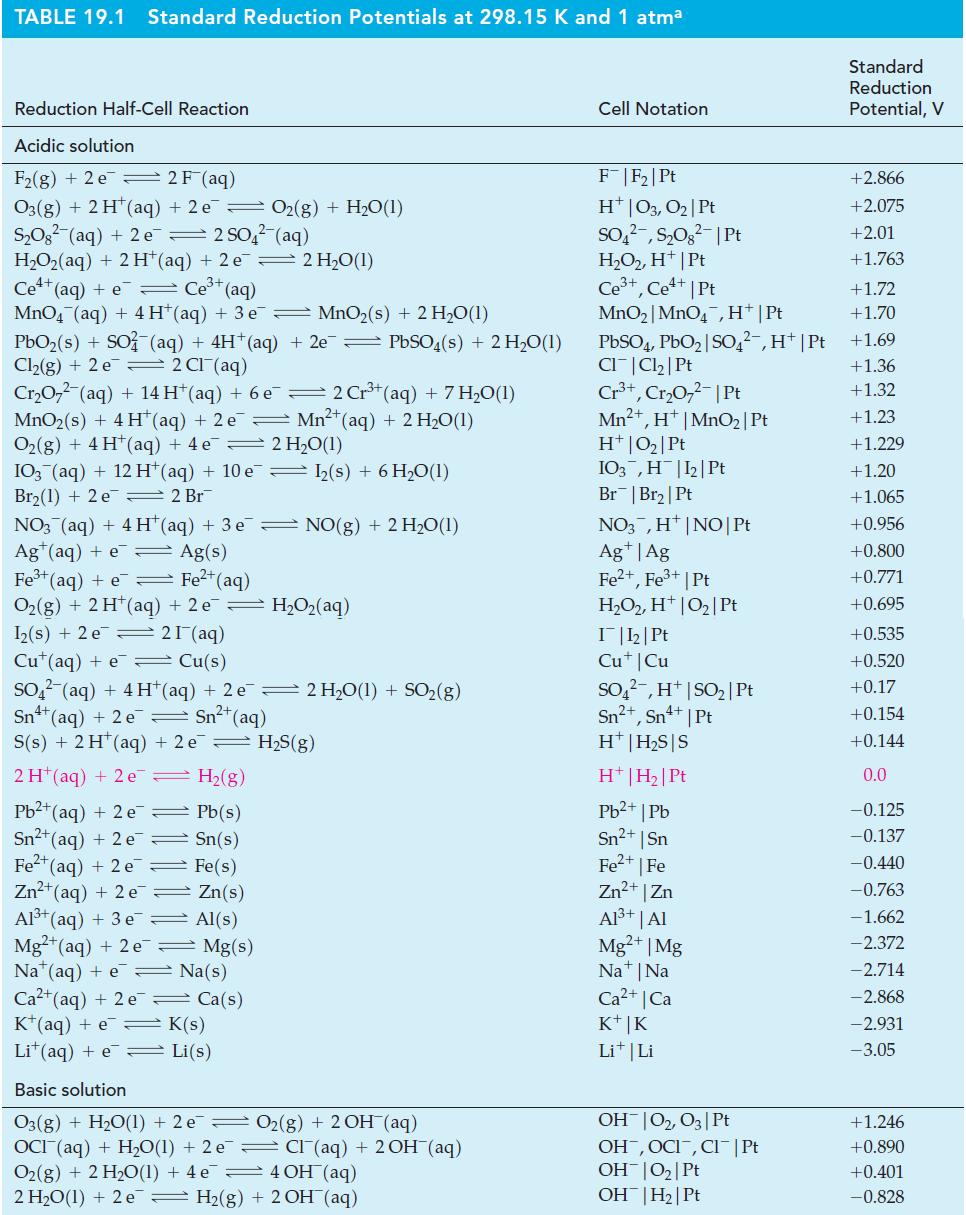
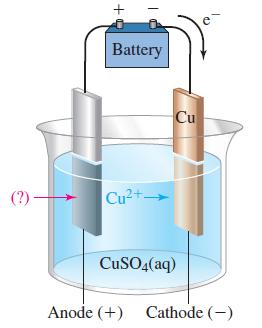
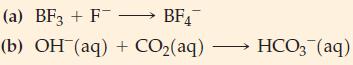
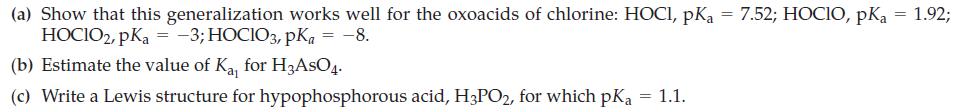
![-AgNO3 solution- [CrO2] = [Br] = 0.010 M 10 (b) [Ag+] 1.0 x 10-5 M [CrO2] = 0.010 M [Br] 5.0 x 10-8 M AgBr(s)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/2/2/375655ef6c7f21e11700722375557.jpg)