![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


![1 Time, S 0 25 50 75 100 150 200 250 [A], M 1.00 0.78 0.61 0.47 0.37 0.22 0.14 0.08 || Time, S 0 25 50 75 100](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/7/448656217d8ca25e1700927446997.jpg)
![Time, S 0 25 50 75 100 150 200 250 [A], M 1.00 0.78 0.61 0.47 0.37 0.22 0.14 0.08 || Time, S 0 25 50 75 100](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/7/476656217f4a90001700927474603.jpg)
![I Time, S 0 25 50 75 100 150 200 250 [A], M 1.00 0.78 0.61 0.47 0.37 0.22 0.14 0.08 || Time, s 0 25 50 75 100](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/7/5016562180dddacb1700927499777.jpg)
![I Time, S 0 25 50 75 100 150 200 250 [A], M 1.00 0.78 0.61 0.47 0.37 0.22 0.14 0.08 || Time, s 0 25 50 75 100](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/7/5286562182838ea81700927525987.jpg)
![Time, S [A], M 1.00 0.78 50 0.61 75 0.47 100 0.37 0.22 0.14 0.08 0 25 150 200 250 || Time, s 0 25 50 75 100](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/7/5956562186b3f62f1700927594050.jpg)
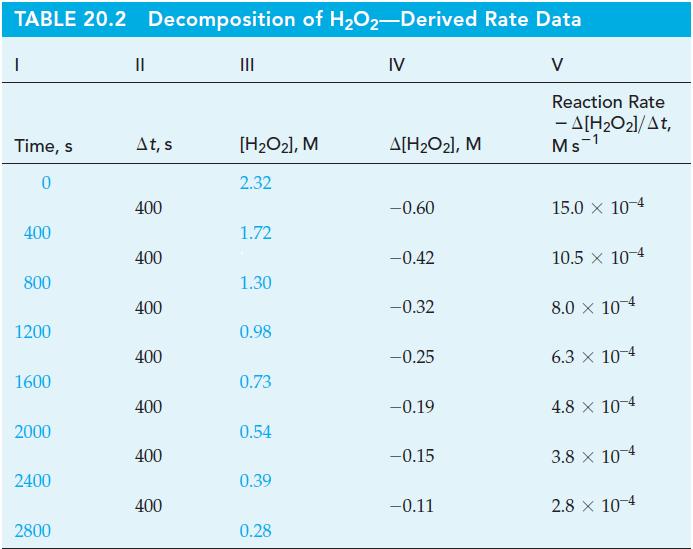
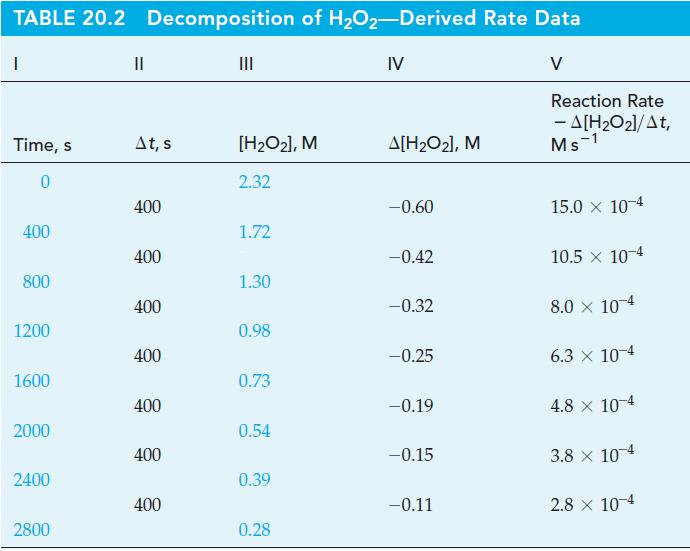
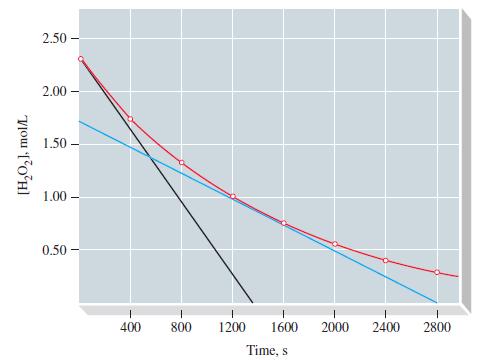
![TABLE 20.3 Kinetic Data for the Reaction: 2 HgCl + CO4- 2Cl + 2 CO + HCl Experiment [HgCl], M 123 2 3](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/1/3/8216561e29d305471700913820443.jpg)
![[H] 0.800 0.400 0.000 -0.400- -0.800 - -1.200 Slope -1.095 L - 1.095 1500 s 600 1500 s -7.30 x 10-45-1 I 1200](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/1/4/5156561e553e2ba41700914514330.jpg)
![[A]t In- [A]o = -kt or In[A] = kt + In[A]o (20.13)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/1/4/5416561e56d69eaf1700914539928.jpg)
![TABLE 20.3 Kinetic Data for the Reaction: 2 HgCl + CO4- 2Cl + 2 CO + HgCl Experiment [HgCl], M [HgCl]1 =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/5/25565620f471fd6b1700925253730.jpg)

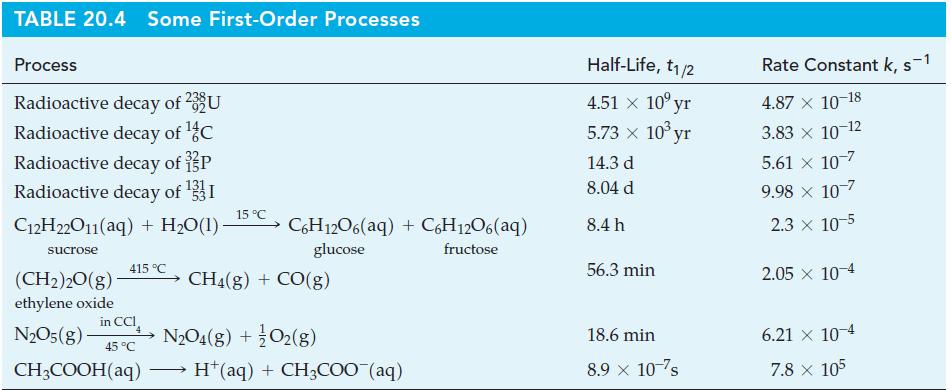
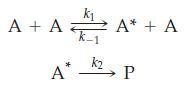
![[Zn(NH3)4]+ (aq) + 2 Ti+ (aq) + e e Zn(s) + 4 NH3(aq) E -1.015 V 2+ Sn+ (aq) + 2 e Ti+ (aq) O2+ (aq) + 2 H+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/8/0/8/697656047f986fcd1700808697206.jpg)
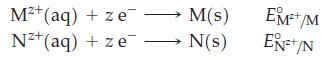
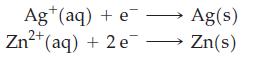
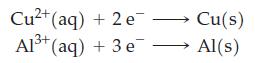
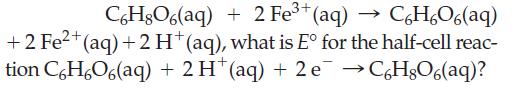
![3 Pt(s) +12 CI (aq) + 2NO3 (aq) + 8 H*(aq) 3[PtCl4] (aq) + 2 NO(g) + 4HO(1)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/8/0/8/0146560454e3f5c81700808013897.jpg)
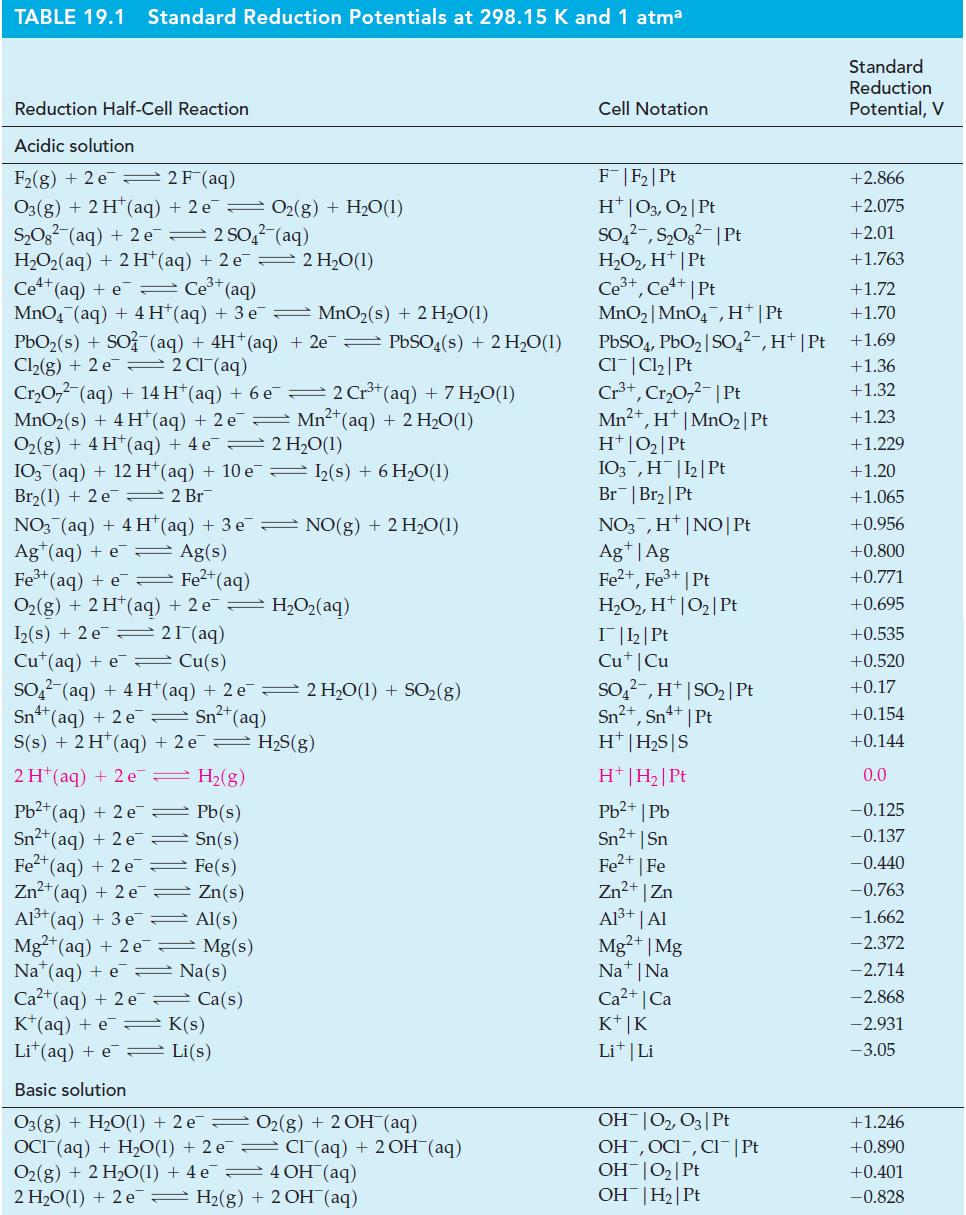
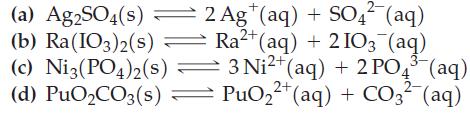
![3+ (a) Ksp = [Fe+][OH-] (b) Ksp = [BiO+][OH] (c) Ksp = [Hg+][r] (d) Ksp = [Pb+][AsO4-1 2+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/2/4/628655eff944c5091700724628060.jpg)
![(a) Kap(CrF3) = 6.6 10-1 (b) Ksp [Au2(C2O4)3] = 1 10-10 (c) Ksp [Cd3(PO4)2] = 2.1 10-33 (d) Ksp (SrF) =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/2/4/723655efff3ab34e1700724723455.jpg)