![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


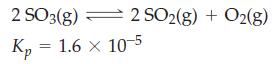
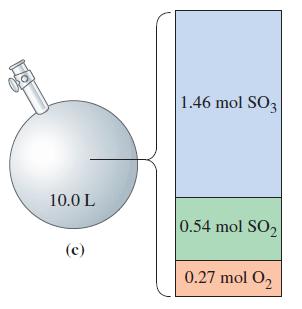
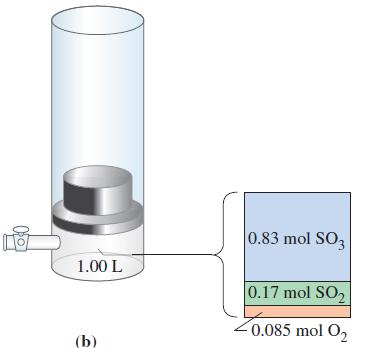
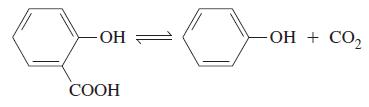
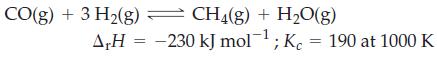
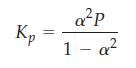
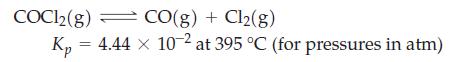
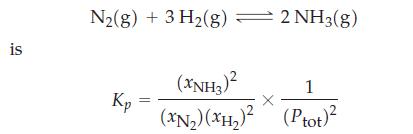
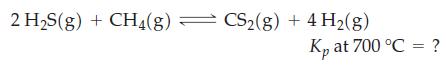
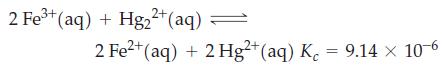
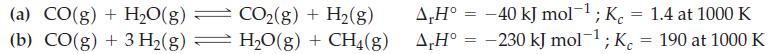
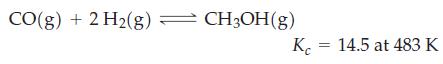
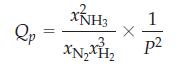
![a = "V(P - P)` P] RT expl](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/4/8/4/480655b5580188e01.png)
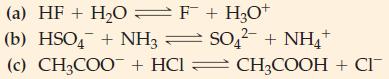
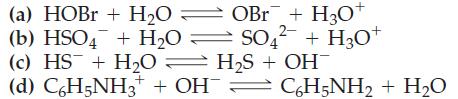

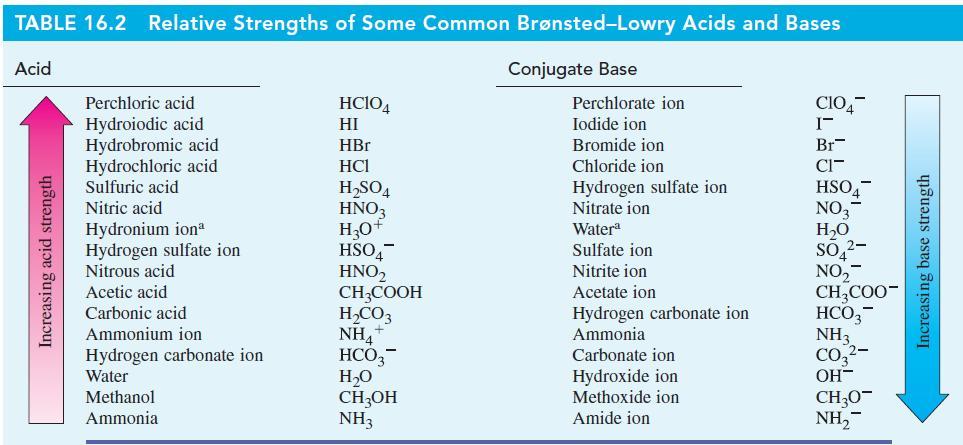
![Kal + HO H3O+ + HOOCCHCOO + HO H3O+ + OOCCHCOO Ka = 2.0 10-6 Calculate [H3O+], [HOOCCHCOO], and](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/5/5/0/340655c56c4d0fd81700550339629.jpg)
![HCO4 + HO HC04 + H0 H3O+ + HC04 H3O+ + C0 An aqueous solution that is 1.05 M HCO4 has pH is [C04] = 5.3 x](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/5/5/0/366655c56de01f6d1700550364764.jpg)
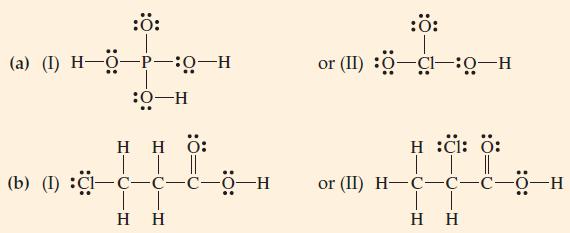
![(a) BF3 + NH3 (b) Cr+ + 6HO F3BNH3 [Cr(HO)6]+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/5/5/0/965655c5935a4ae31700550964428.jpg)
![(a) Al(OH)3 + OH - [Al(OH)4] (b) SnCl4 + 2 CI [SnC16]-](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/5/5/0/984655c5948b2fe91700550983479.jpg)