![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


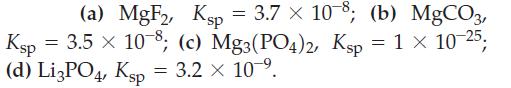
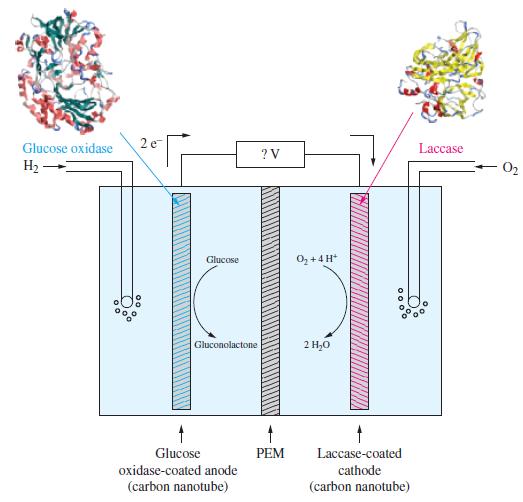
![(a) [Pb+] = []; (b) [Pb+] = Ksp of PbI; (c) [Pb+] = Ksp of PbI2; (d) [Pb+] = 0.5[1].](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/3/8/897655f375159c5d1.png)
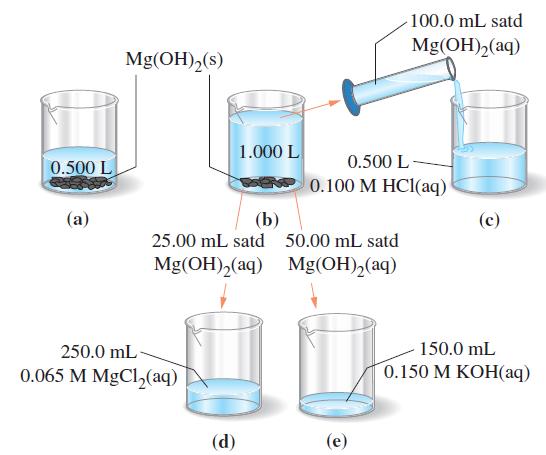
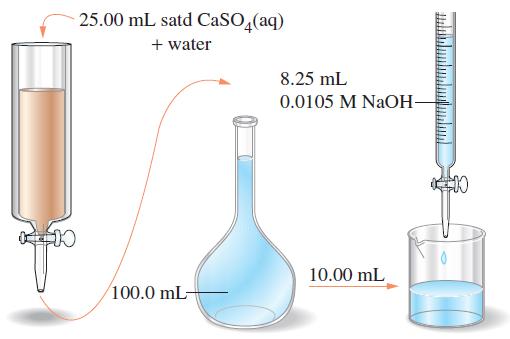
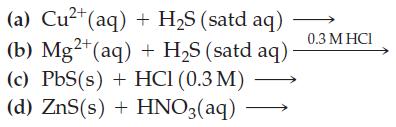
![[[Ag (SO3)2]] = 0.048 M, [SO3] = 0.76 M, and [I] = 2.0 M?](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/2/9/816655f13d8d12951.png)
![Cu+ (aq) + 4 NH3(aq) [Cu(NH3)4]+ (aq) Kf = 1.1 X 1013](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/7/2/9/754655f139a68ef31700729754083.jpg)
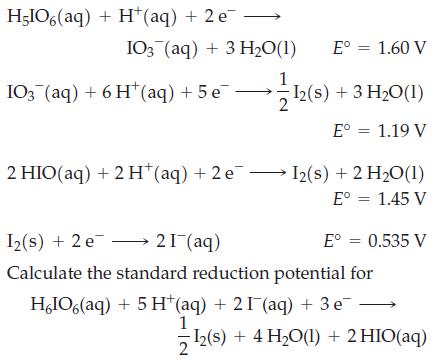
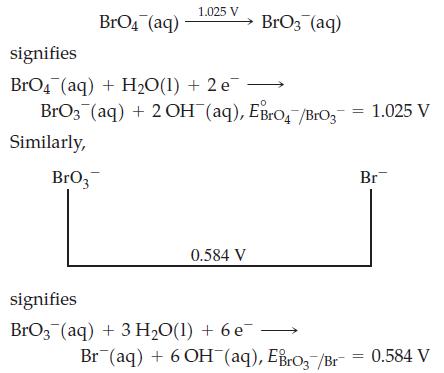
![Basic solution ([OH-] = 1 M): 1.025 V (?) BrO4 BrO3 Bro (?) 0.584 V Br (?) (?) Br](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/8/9/6/36165619e69ec5cb1700896359617.jpg)
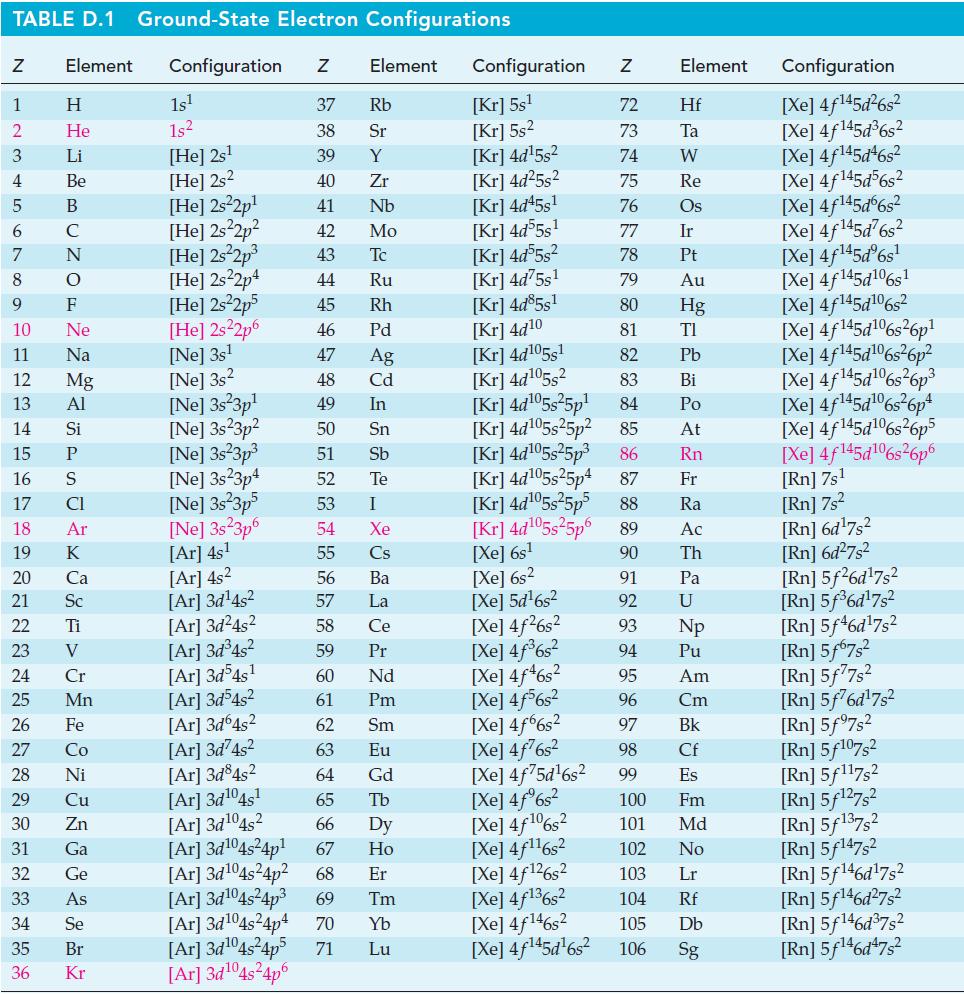
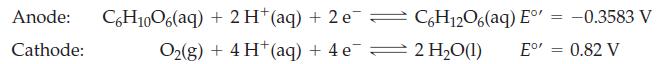
![[HO], mol/L 2.50- 2.00- 1.50 1.00 0.50- 1 400 1 800 1 1200 1600 Time, s 2000 2400 2800](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/4/81465620d8e297281700924813659.jpg)
![TABLE 20.3 Kinetic Data for the Reaction: 2 HgCl + CO4- 2 Cl + 2 CO + H2Cl Experiment 1 123 2 3 [HgCl], M =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/0/5/3116561c15fe3ed61700905310844.jpg)
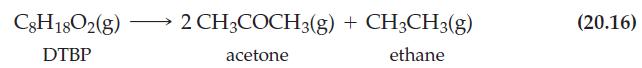
![TABLE 20.3 Kinetic Data for the Reaction: 2 HgCl + CO4- 2Cl + 2 CO + HCl Experiment 1 12 2 3 [HgCl], M](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/5/636656210c46b9741700925631178.jpg)
![TABLE 20.6 Time, min 0 5055 10 15 25 Kinetic Data for Example 20-8 [A], M In [A] 1/[A] 1.00 0.00 1.00 0.63](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/0/5/9986561c40e97bca1700905996734.jpg)
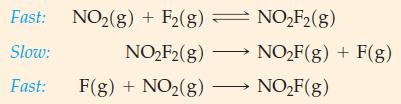
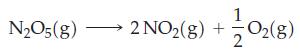
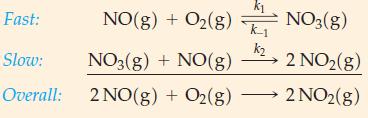
![Expt 1 2 3 4 [A], M 0.185 0.185 0.370 0.370 [B], M 0.133 0.266 0.133 0.266 Initial Rate, M s-1 3.35 x 10-4](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/5/950656211febef311700925948118.jpg)
![Expt 123 2 3 [A], M 0.50 1.50 3.00 [B], M 1.50 1.50 3.00 Initial Rate, M min-1 4.2 10- 1.3 x 10-2 5.2 x 10-2](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/6/039656212571c3561700926037197.jpg)
![Expt 123 Initial [NO], M 0.0125 0.0125 0.0250 Initial [Cl], M 0.0255 0.0510 0.0255 Initial Rate of Reaction,](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/6/139656212bb7b5711700926137026.jpg)
![Expt 1 2 3 45 Initial [A], M 1.40 0.70 0.70 1.40 0.70 Initial [B], M 1.40 1.40 0.70 1.40 0.70 [C], M 1.00](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/9/2/6/199656212f7858a41700926196970.jpg)