![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


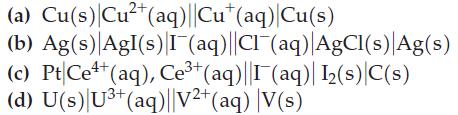
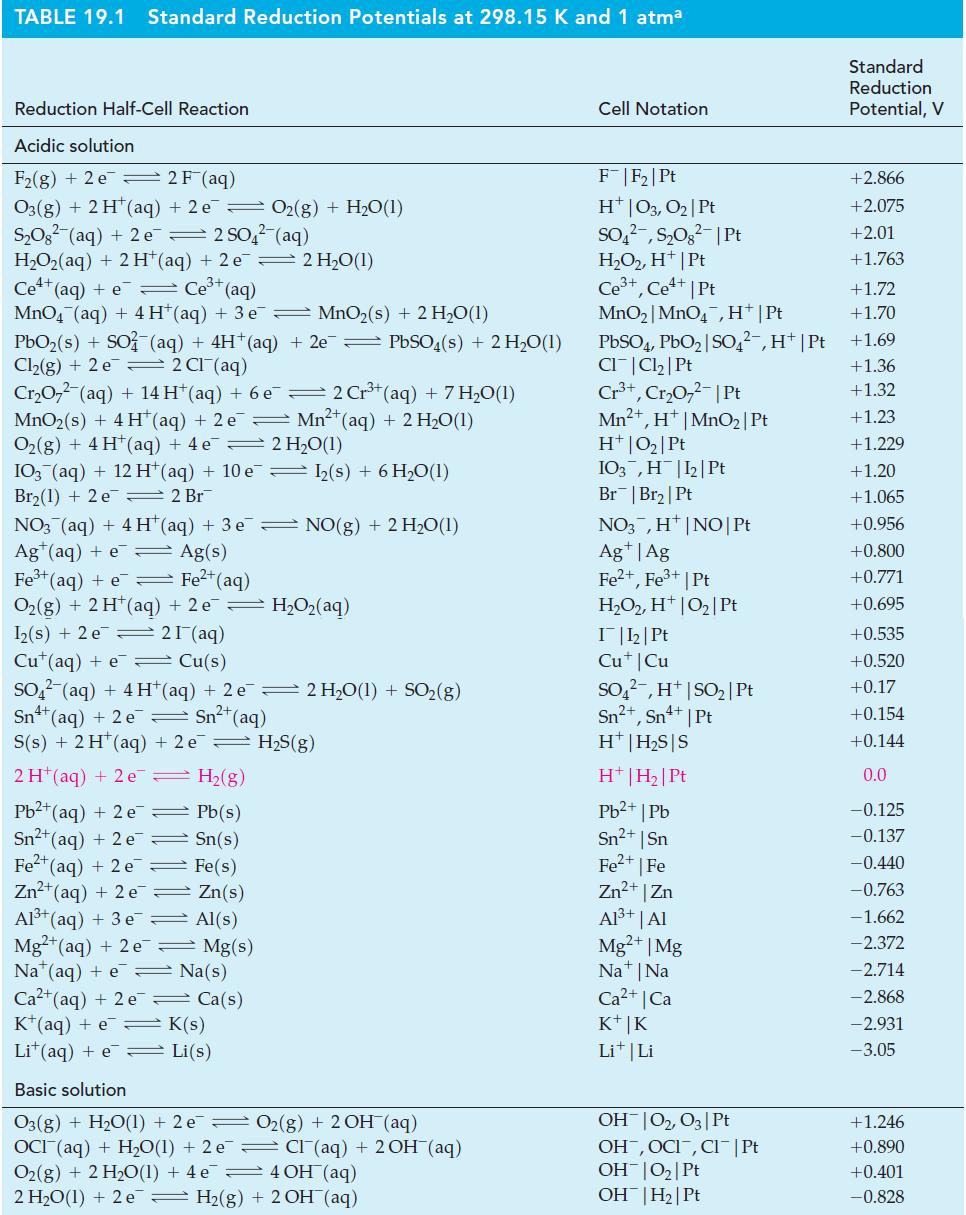
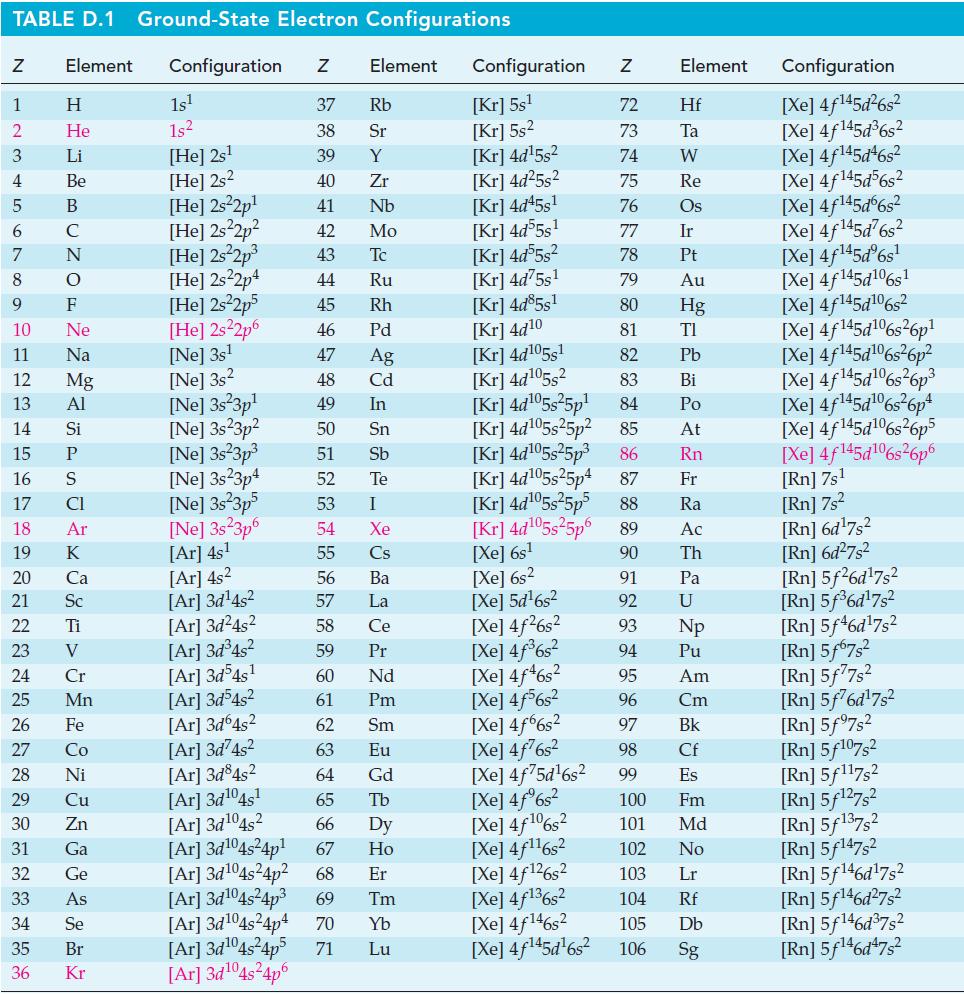
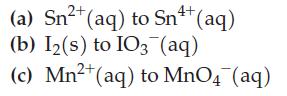
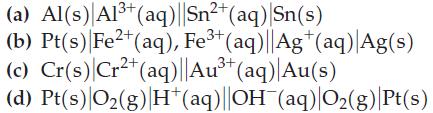
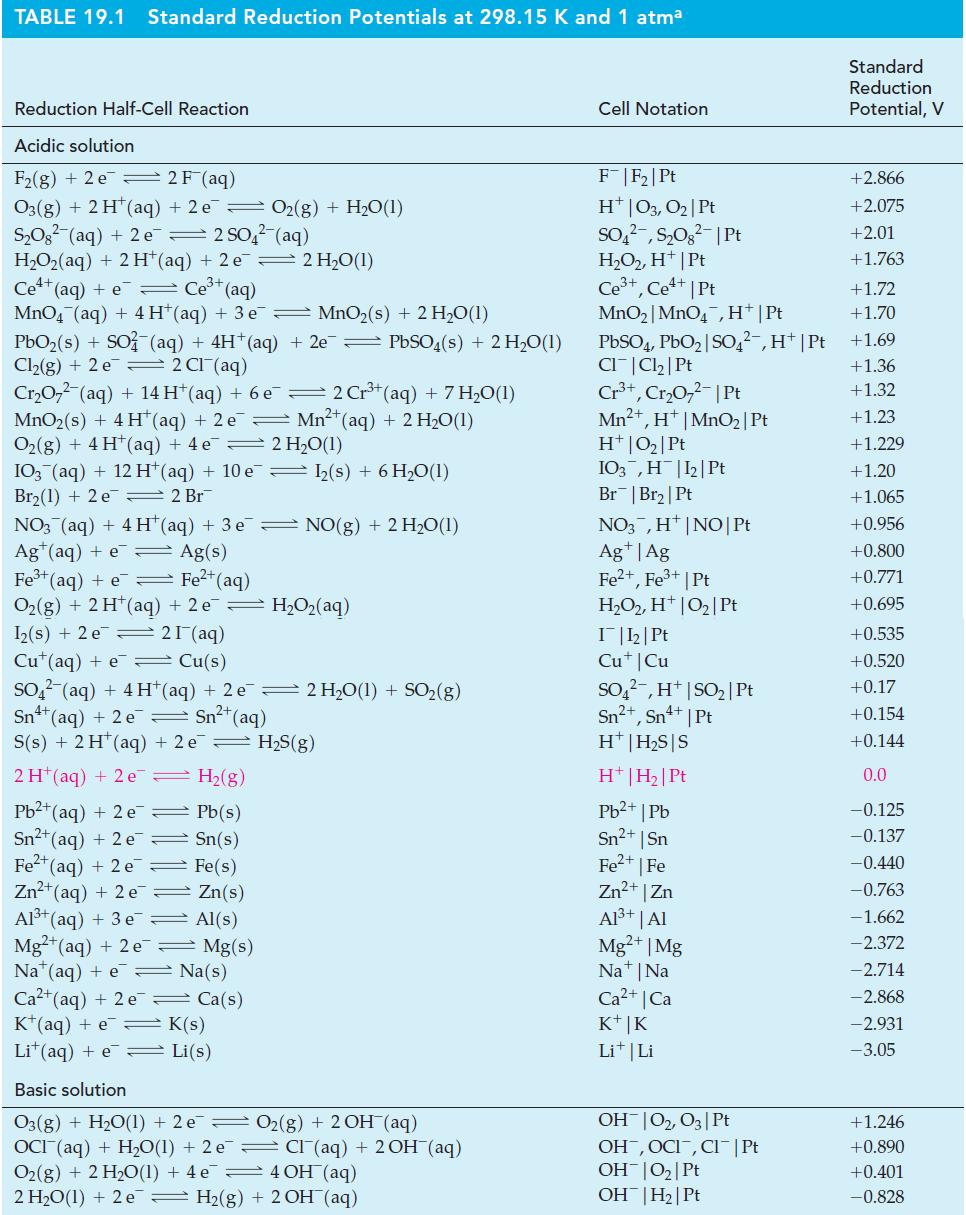
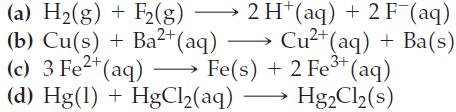
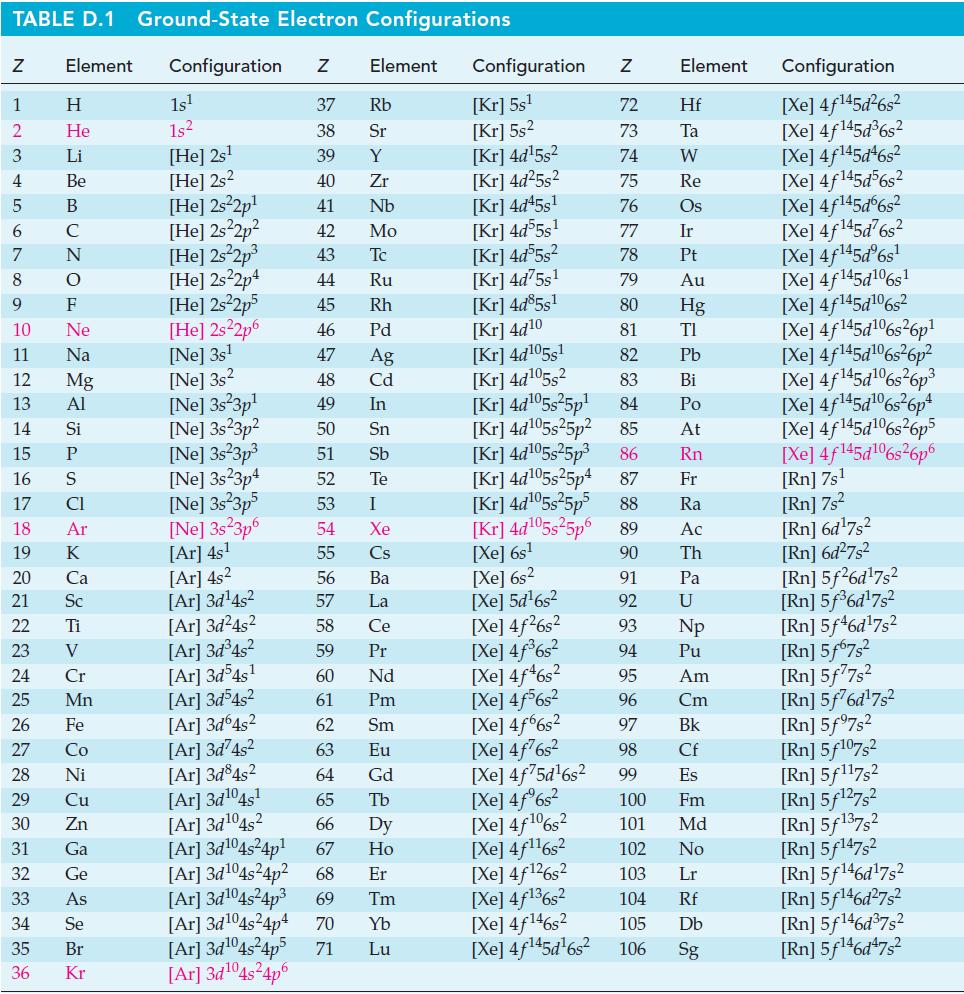
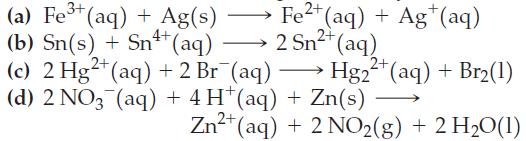
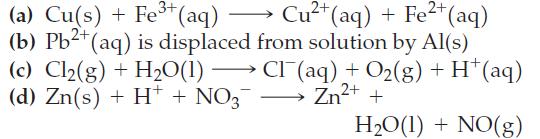
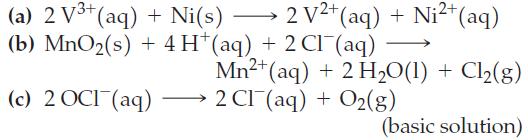

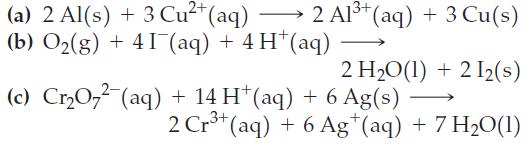
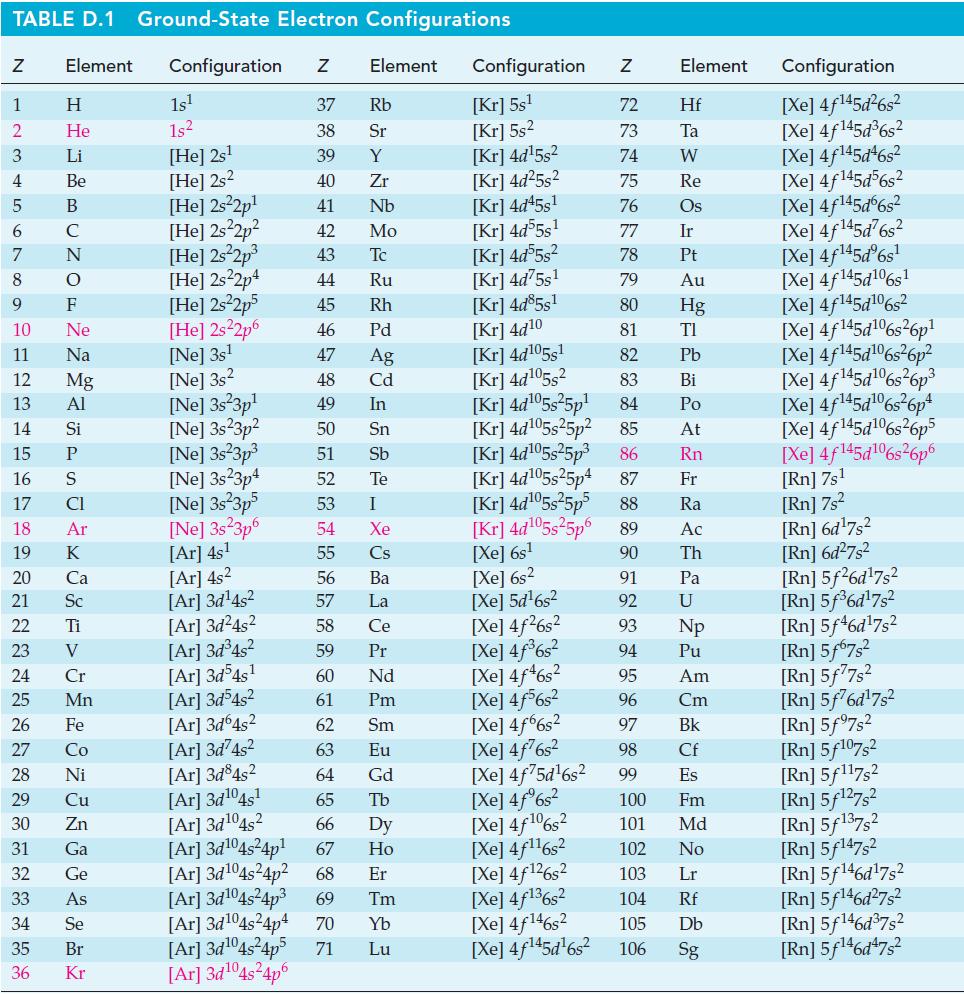
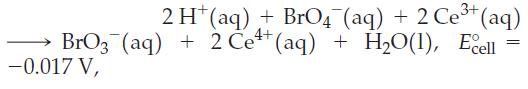
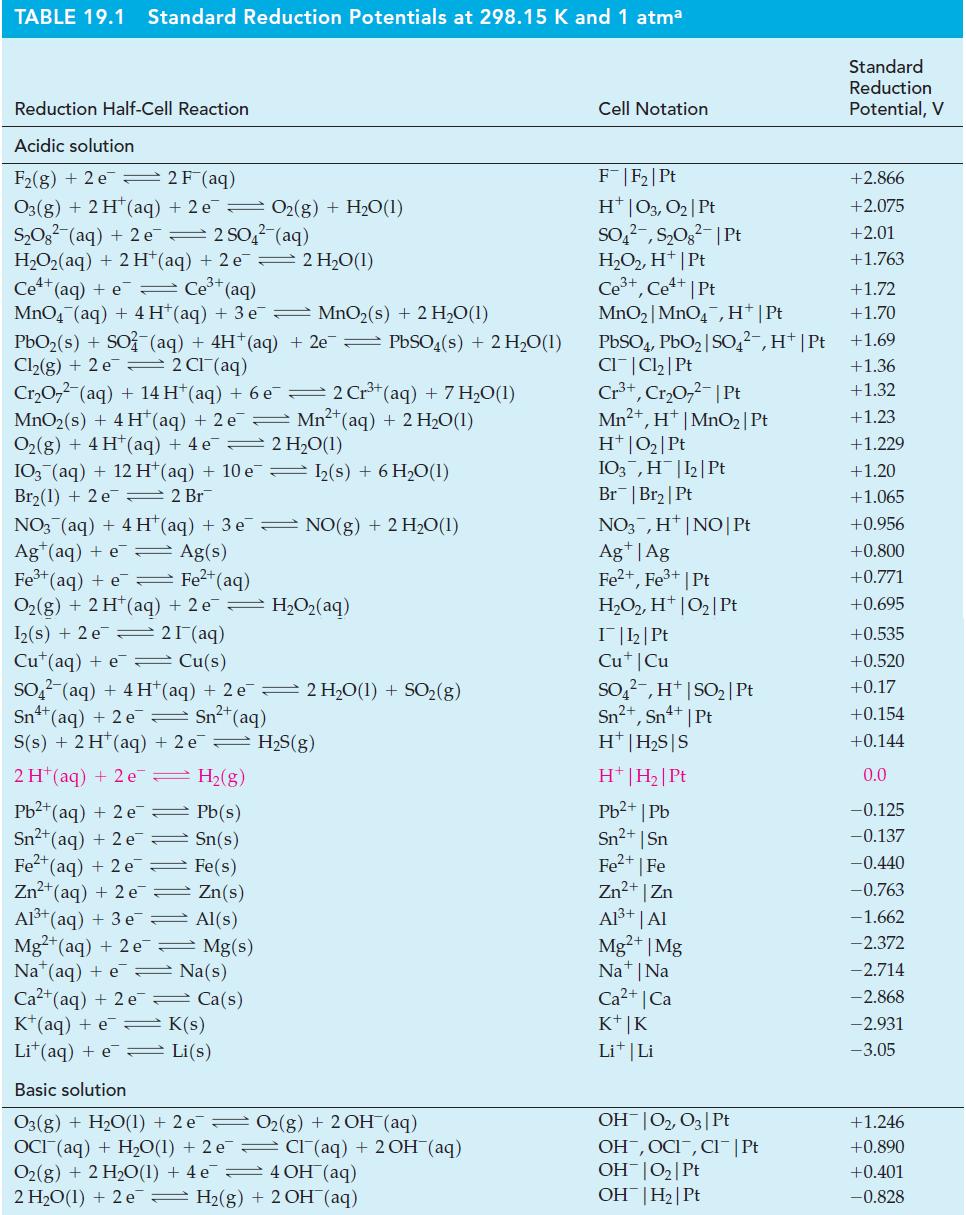
![= [BrO4] = [Ce4+] = 0.675 M, [BrO3] = [Ce+] = 0.600 M and pH = 1?](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/8/1/1/8856560546daabf92.png)
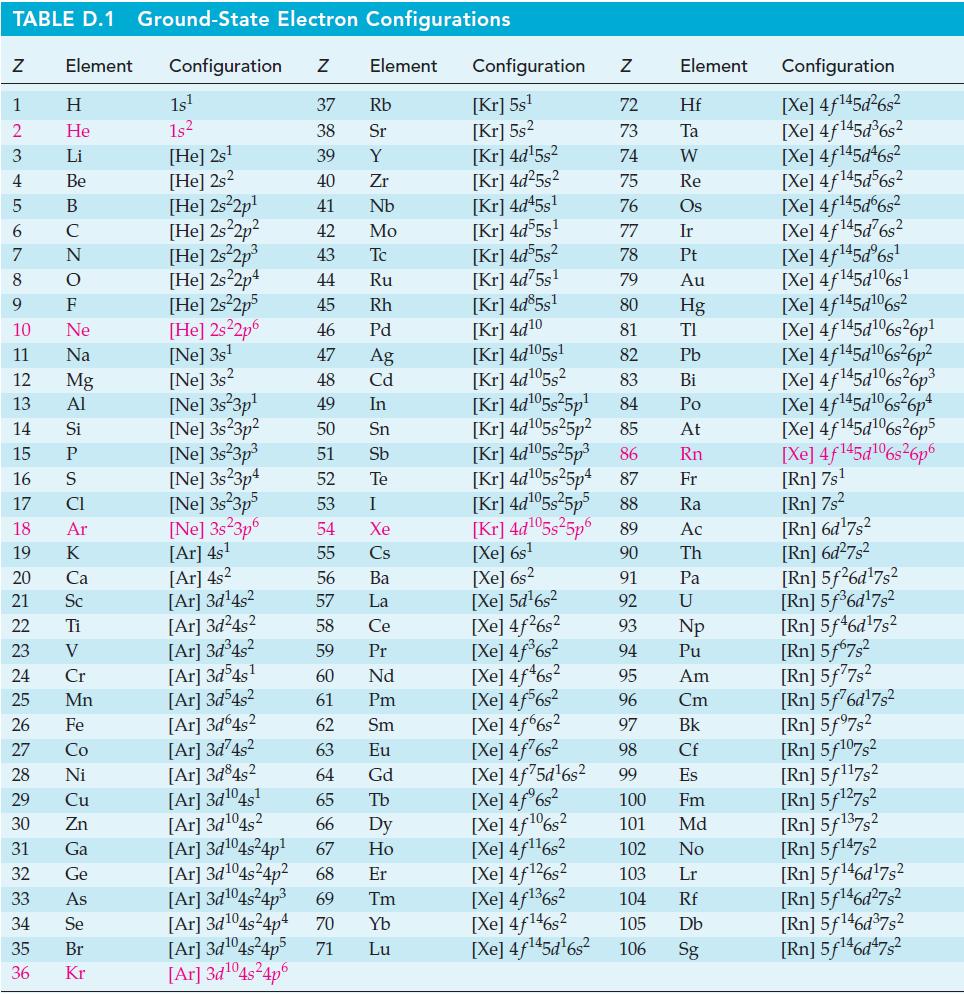
, OH](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/8/1/3/79965605be75484e2.png)
![H(g, 1 bar) Pt Anode [H+] = x M Voltmeter Salt bridge KNO3(aq) Cathode [H+] = 1 M H(g, 1 bar) Pt](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/8/1/3/285656059e5c56e91700813285372.jpg)
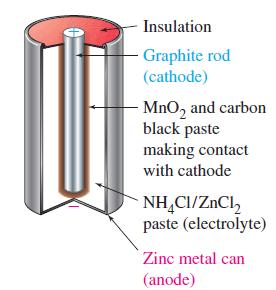
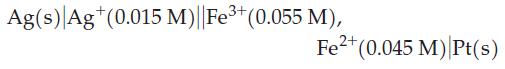
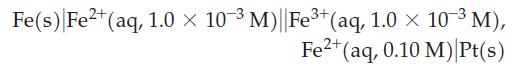
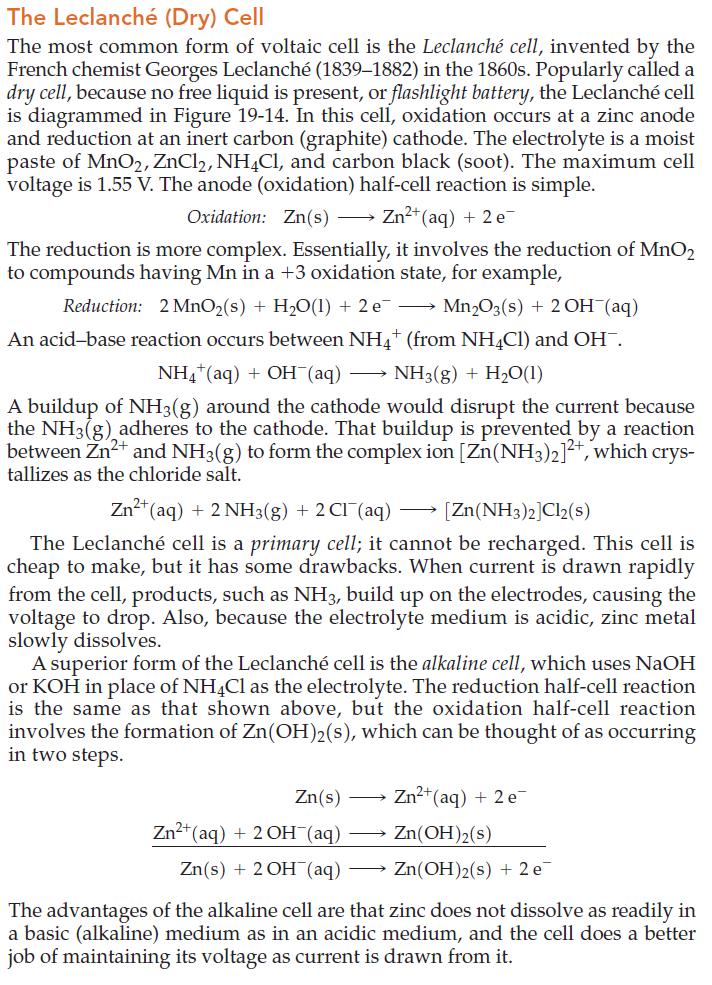
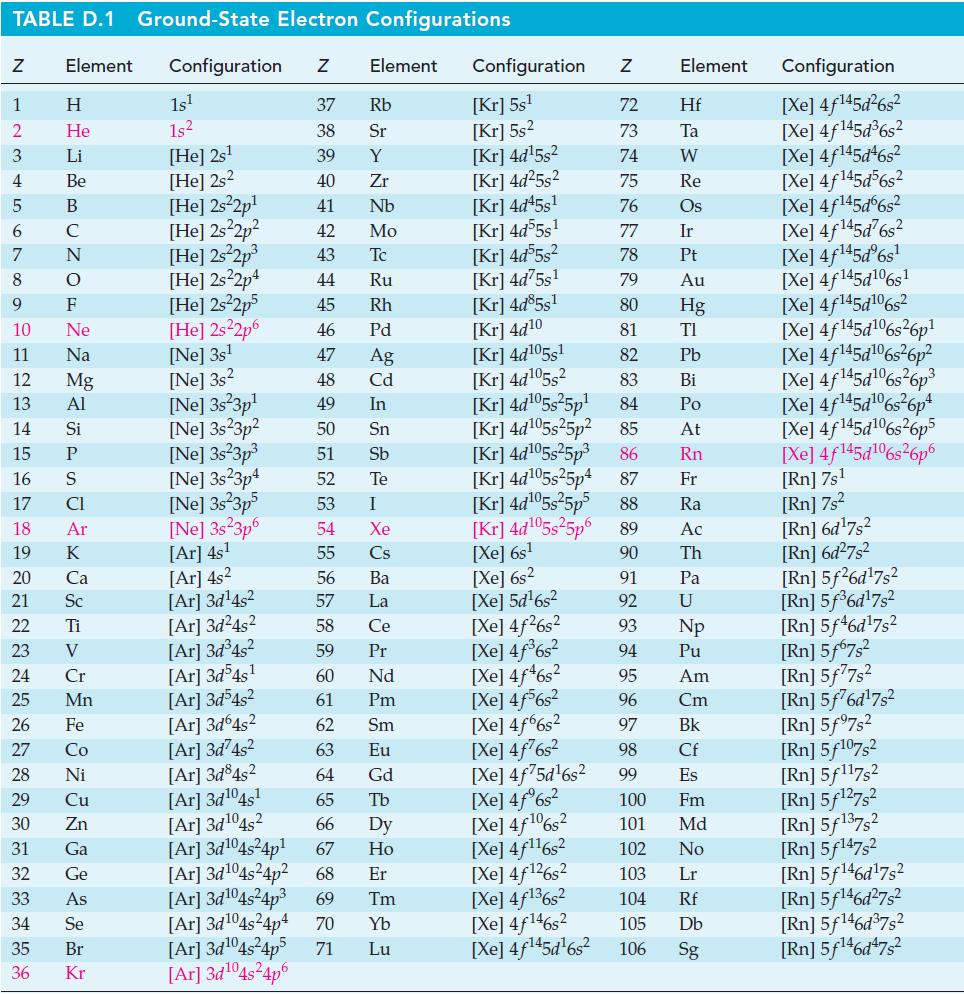
![(a) 2 HO(1) 2 H(g) (b) Zn(s) + Fe+ (aq) (c) 2 Fe+ (aq) + I(s) (d) Cu(s) + Sn+ (aq) + O(g) [in 1 M H*(aq)] 2+](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/8/2/0/0166560743011a6e2.png)