![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


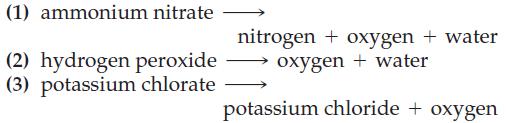
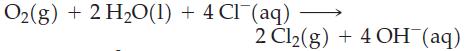
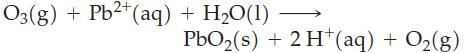

![NO3(aq) + Zn(s) + OH(aq) + HO(1) [Zn(OH)4] (aq) + NH3(g)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/2/6/1/432656730782fa451701261431664.jpg)

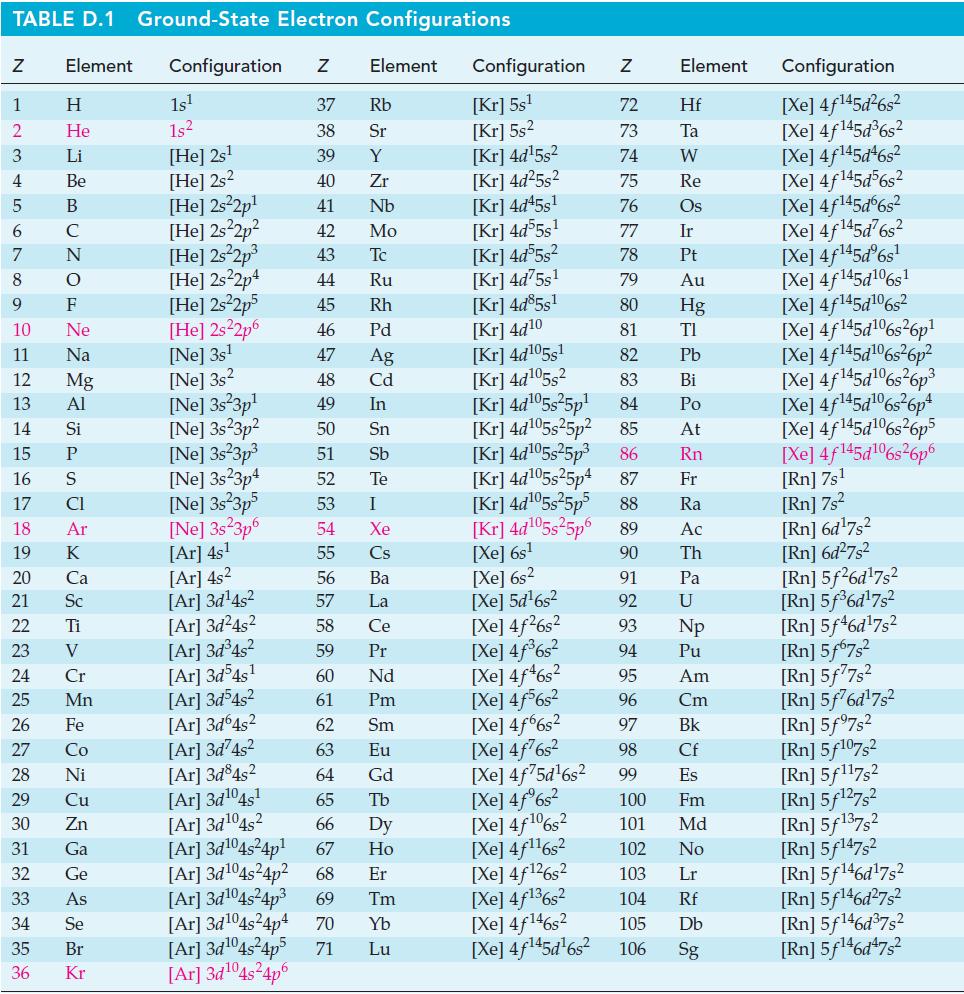
![Acidic solution ([H+] = 1 M): +6 +5 -0.22 V SO4- -S06- 0.158 V SO4-- 0.564 V -0.936 V Basic solution ([OH-] =](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/3/2/0/659656817d357a171701320657675.jpg)
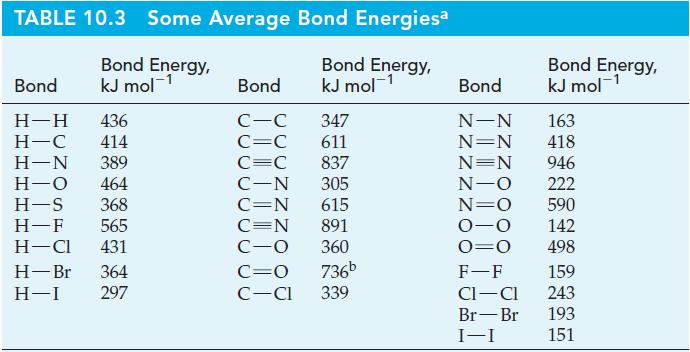
![Acidic solution ([H+] = 1 M): +5 +4 +3 +2 +1 0 -1 0.803 V 1.065 V 0.996 V 1.591 V 1.766 V -1.87 V 1.275 V](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/3/2/1/0056568192de8cfc1701321004592.jpg)
![Acidic solution ([H+] = 1 M): +7 +5 CIO4 1.189 V CIO4 CIO3 0.374 V Basic solution ([OH-] = 1 M): +7 +5 1.181](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/3/2/2/23065681df67f4d71701322229081.jpg)